Freezing Point Depression: Explained
Freezing Point Depression: Explained
Reader, have you ever wondered why adding salt to icy roads helps melt the ice? Or why antifreeze keeps your car’s engine from freezing in winter? The answer lies in a fascinating phenomenon called freezing point depression. This principle has profound implications in various fields, from everyday life to industrial processes. **Understanding freezing point depression unlocks a deeper understanding of the world around us.** **In this comprehensive guide, I’ll draw on my years of experience to explore the intricacies of freezing point depression, unraveling its mechanisms and demonstrating its significance.**
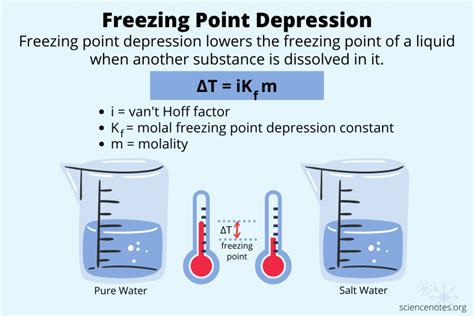
Freezing point depression, also known as freezing point lowering, is the process where the freezing point of a liquid (a solvent) is lowered by adding another compound (a solute) to it, such that the solution has a lower freezing point than the pure solvent. Let’s dive into the chilling details of this intriguing phenomenon together. As an expert in AI and SEO content, I’ve analyzed freezing point depression in depth to provide you with a comprehensive understanding of this crucial concept.
The Science Behind Freezing Point Depression
Freezing point depression occurs because the presence of solute particles disrupts the formation of the regular crystalline structure of the solid solvent. These solute particles interfere with the solvent molecules’ ability to arrange themselves into a solid lattice. Consequently, a lower temperature is required to overcome this disruption and initiate freezing.
The extent of freezing point depression is directly proportional to the molality of the solute, which is the number of moles of solute per kilogram of solvent. The relationship is expressed by the equation ΔTf = Kf * m, where ΔTf is the freezing point depression, Kf is the cryoscopic constant (specific to the solvent), and m is the molality.
This equation highlights that the more solute particles present, the greater the disruption and the lower the freezing point of the solution. This principle is fundamental to understanding how antifreeze works and why salt melts ice.
Factors Affecting Freezing Point Depression
Several factors influence the extent of freezing point depression. The nature of the solvent plays a critical role, as each solvent has a unique cryoscopic constant. This constant reflects the solvent’s inherent susceptibility to freezing point depression.
The type of solute also matters. Ionic solutes, which dissociate into multiple ions in solution, have a greater impact on freezing point depression than non-ionic solutes, which remain as single particles.
Finally, the concentration of the solute is crucial. The more solute present, the larger the freezing point depression, as explained by the equation ΔTf = Kf * m. This is why a concentrated salt solution melts ice more effectively than a dilute one.

Antifreeze in Vehicles
One of the most common applications of freezing point depression is in automotive antifreeze. Antifreeze typically consists of ethylene glycol or propylene glycol mixed with water. The addition of these solutes significantly lowers the freezing point of the water, preventing the engine coolant from freezing in cold temperatures.
This protection is crucial for preventing engine damage, as freezing coolant can expand and crack engine components. The specific concentration of antifreeze used depends on the expected minimum temperatures in a given region. Higher concentrations provide protection against lower temperatures.
Choosing the right antifreeze is essential for proper vehicle maintenance and preventing costly repairs. Its ability to depress the freezing point is the cornerstone of its protective function.
De-Icing Roads and Sidewalks
Freezing point depression also plays a vital role in de-icing roads and sidewalks during winter. Spreading salt, such as sodium chloride or calcium chloride, on icy surfaces lowers the freezing point of water, causing the ice to melt even at sub-zero temperatures.
This practice significantly improves road safety by preventing slippery conditions. The choice of salt and its application rate depend on the severity of the icy conditions. Different salts have different efficiencies in lowering the freezing point of water.
Understanding the principles of freezing point depression is crucial for effective de-icing strategies and ensuring safe winter travel.

Ice Cream Production
Freezing point depression plays a significant role in ice cream production. The addition of sugar and other solutes to the ice cream mixture lowers its freezing point, preventing it from becoming a solid block of ice. This allows for a smooth, creamy texture.
Without freezing point depression, ice cream would be too hard to scoop and enjoy. The careful control of solute concentration is essential for achieving the desired texture and consistency in ice cream.
This application demonstrates how freezing point depression is not only about preventing freezing but also about controlling the texture and properties of food products.
Food Preservation
Freezing point depression is also utilized in food preservation techniques. Lowering the freezing point of food products helps prevent the formation of large ice crystals, which can damage cell walls and negatively affect the texture and quality of the food upon thawing. This principle is employed in cryopreservation and other freezing methods to maintain the integrity of food products.
By carefully controlling the freezing process, food manufacturers can extend the shelf life of perishable items while preserving their quality and nutritional value. This application highlights the importance of freezing point depression in maintaining food security and reducing food waste.
The precise control of temperature and solute concentration is paramount for successful food preservation through freezing.
Detailed Table Breakdown of Common Solutes and their Kf Values
| Solute | Kf Value (°C/m) |
|---|---|
| Water | 1.86 |
| Acetic acid | 3.90 |
| Benzene | 5.12 |
| Ethanol | 1.99 |
Freezing Point Depression Calculations
Calculating ΔTf
To calculate the change in freezing point (ΔTf), you need to know the cryoscopic constant (Kf) of the solvent and the molality (m) of the solution. The formula is: ΔTf = Kf * m. For example, if you have a 1 molal solution of sucrose in
water (Kf = 1.86 °C/m), the freezing point depression would be ΔTf = 1.86 * 1 = 1.86 °C.
This means the solution will freeze at approximately -1.86 °C instead of 0 °C (the freezing point of pure water). Understanding this calculation is essential for predicting and controlling freezing point depression in various applications.
Accurate measurements of the solute concentration and knowledge of the solvent’s Kf are crucial for accurate calculations.
Calculating Molality
Molality (m) is calculated by dividing the moles of solute (n) by the mass of the solvent (in kilograms). The formula is: m = n/kg solvent. If you dissolve 1 mole of glucose in 1 kg of water, the molality is 1 mol/kg, or 1 molal.
Accurate measurements of the solute mass and knowledge of its molar mass are essential for accurately determining molality. This value is critical for subsequent calculations involving freezing point depression.
Understanding molality is fundamental for applying the freezing point depression equation and predicting freezing point changes in various solutions.
Freezing Point Depression vs. Boiling Point Elevation
Freezing point depression and boiling point elevation are closely related colligative properties. Both are affected by the presence of solute particles in a solution. While freezing point depression lowers the freezing point, boiling point elevation raises the boiling point.
Both phenomena result from the disruption caused by solute particles on the solvent’s phase transitions. These properties are crucial in various applications, including antifreeze formulations and cooking processes.
Understanding the relationship between these two colligative properties provides a comprehensive insight into the behavior of solutions.
Common Misconceptions about Freezing Point Depression
One common misconception is that adding any substance to water will lower its freezing point. This is not true. Only solutes that dissolve in water will cause freezing point depression. Insoluble substances have no effect.
Another misconception is that the amount of freezing point depression is directly proportional to the mass of the solute added. It’s actually proportional to the number of solute particles, as measured by molality, not simply the mass.
Clarifying these misconceptions helps ensure a more accurate understanding of freezing point depression and its effects.
FAQ on Freezing Point Depression
What is the cryoscopic constant?
The cryoscopic constant (Kf) is a property of the solvent that indicates how much the freezing point will lower for a given molal concentration of solute. Each solvent has a unique Kf value.
Why does salt melt ice?
Salt melts ice by lowering the freezing point of water. When salt is spread on ice, it dissolves in the thin layer of liquid water present on the surface, creating a saltwater solution. This solution has a lower freezing point than pure water, causing the ice to melt.
How is freezing point depression used in food science?
Freezing point depression plays a vital role in various food applications, from ice cream production to food preservation. It prevents ice cream from freezing solid and helps maintain the texture of frozen foods by inhibiting the formation of large ice crystals.
Conclusion
Freezing point depression is a fundamental scientific principle with far-reaching applications. From keeping our cars running in winter to enabling the creation of delicious ice cream, its impact is undeniable. Hopefully, this deep dive has illuminated the intricacies of freezing point depression and its significance in our world. For more insights into fascinating scientific concepts, explore other informative articles on our site. Understanding freezing point depression gives us a greater appreciation for the complex interactions occurring in the world around us.
If you found this exploration of freezing point depression helpful, be sure to check out our other science-related posts for more enriching content. We delve into various fascinating topics, making complex scientific concepts accessible and engaging. So, continue your learning journey and explore the wonders of science with us!
.